GU research helps unlock pathway to fight parasites - The Spokesman-Review
GU research helps unlock pathway to fight parasites - The Spokesman-Review |
- GU research helps unlock pathway to fight parasites - The Spokesman-Review
- Stronger treatments could cure Chagas disease - Medical Xpress
- Malawi becomes the second country in sub-Saharan Africa to eliminate lymphatic filariasis (elephantiasis) - PRNewswire
- 3P Biopharmaceuticals and SpyBiotech Sign Vaccine Manufacturing Agreement - BioPharm International
- How deadly parasites 'glide' into human cells - Science Daily
| GU research helps unlock pathway to fight parasites - The Spokesman-Review Posted: 28 Oct 2020 02:06 PM PDT  A study led by Gonzaga University professor Jennifer Shepherd raises the prospects for developing new drugs to kill parasites, which have sickened an estimated 1.5 billion people worldwide. Her work targets parasitic helminths, a class including roundworms and tapeworms that live in soil and colonize human guts through dirty water. Parasitic diseases can have significant and chronic consequences for child development. Shepherd, chair of Gonzaga's chemistry and biochemistry department, said the new study found that the parasites have a unique enzyme variant allowing them to synthesize rhodoquinone, a molecule needed to survive in the low-oxygen space of the human gut and intestines. That enzyme now can be targeted for new treatments. "We've been trying to find a difference, something that we can selectively target that only parasites have that humans don't, and we have found that now," said Shepherd, who has studied the biosynthesis of rhodoquinone for nearly 20 years. "If we want to develop a drug to kill parasites, we can target now this enzyme variant. We can try to shut it down so it can't work and it can't make this molecule, rhodoquinone. Without rhodoquinone, they die when they're inside the host." Parasites can live in both oxygen environments and spaces with little to no oxygen. Once inside a host, they switch to an alternative metabolic pathway – a type of anaerobic or oxygen-independent metabolism – for generating energy to survive. A global team of researchers including Shepherd seeks to advance understanding of the mechanisms involved in how the parasites make the switch. Mammals, who use aerobic respiration, don't make or need rhodoquinone. Rather, humans use ubiquinone, also named coenzyme Q10 or CoQ10, for aerobic metabolism. Parasites can make both of molecules. "We've figured out that the parasites have acquired this enzyme variant that humans don't have in order to make rhodoquinone," Shepherd said. "To make RQ, parasites need a different enzyme than is needed to make CoQ10." Parasites affect humans, domestic animals and livestock. Parasite-related illness is considered a neglected tropical disease because large funding typically isn't given for research to study or develop new anti-parasitic drugs, she said. Very few therapeutics are available. "In the last 30 or 40 years, I think there's only been three new ones developed, and under the current ones, the parasites are gaining resistance to them; that just happens naturally," said Shepherd, who will continue work with the team to find future treatments. "We really need a new kind of anti-parasitic drug. The current ones that are in use have a number of side effects because they target energy systems or metabolisms that are present in both hosts and the parasites, so humans also can be affected by the drugs negatively. "That's why we're trying to find a unique pathway that only the parasites have and try to shut that down so that it won't negatively affect the host. Something that's more selective hopefully would be more difficult to gain resistance to. That's why we're interested in that area." Shepherd also has a personal interest, after spending much time in Ghana, from where she adopted three kids who have been home with her family for 10 years. She's seen many parasitic infections firsthand during her Africa visits. Such infections are common in less-developed countries, but parasites are found in some North American locations, Shepherd said. "The infections are in North America, and all over the world, but highly prevalent in third-world countries where they have poor water quality because a lot of these parasitic eggs grow in contaminated water and soil." The study's leaders also included Gustavo Salinas, a professor at Universidad de la República in Uruguay, and Andrew Fraser, professor of molecular genetics at the University of Toronto's Donnelly Centre for Cellular and Biomolecular Research. Funding came from the Canadian Institutes for Health Research and Agencia Nacional para la Innovación y la Investigación ANII in Uruguay. |
| Stronger treatments could cure Chagas disease - Medical Xpress Posted: 29 Oct 2020 05:49 AM PDT 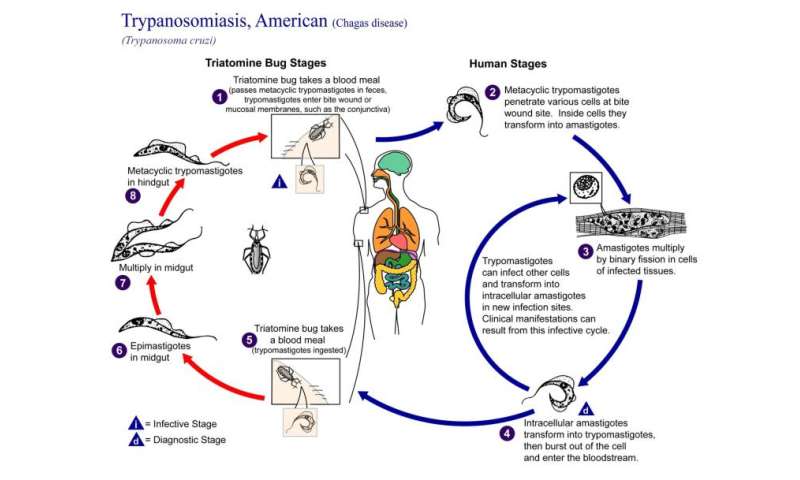 Researchers in the University of Georgia's Center for Tropical and Emerging Global Diseases have found that a more intensive, less frequent drug regimen with currently available therapeutics could cure the infection that causes Chagas disease, a potentially life-threatening illness affecting up to 300,000 people in the United States. Trypanosoma cruzi is a single-celled parasitic organism that causes Chagas disease. At least 6 million people are infected by T. cruzi, mostly in South America. Current drug therapies have been ineffective in completely clearing the infection and are associated with severe adverse side effects. A single dose of benznidazole has been shown to be highly effective in killing more than 90% of parasites. However, after a CTEGD team found some of the parasites enter into a dormancy stage, the researchers hypothesized that an intermittent treatment schedule could be effective. "In this system we can see what a single dose of drug does," said Rick Tarleton, Regents' Professor in UGA's department of cellular biology. "Does it make sense to give a drug twice daily when the remaining dormant parasites are insensitive to it?" The investigators found that giving as little as two-and-a-half times the typical daily dose of benznidazole, once per week for 30 weeks, completely cleared the infection, whereas giving the standard daily dose once a week for a longer period did not. "Current human trials are only looking at giving lower doses over a shorter time period, which is the exact opposite of what we show works," said Tarleton. Since Tarleton's team worked with a mouse model, how this change in treatment regimen will translate in humans is yet unknown, as are any potential side effects of the higher doses. Adverse reactions already are a problem with current treatments; the hope is that side effects from a less frequent dosage would be more tolerable. Significant challenge Assessing the success of treatments in Chagas disease is a significant challenge. Tissue samples from infected organisms might not be representative of the entire organ or animal, since low numbers of persistent, dormant parasites can be difficult to detect. Therefore, Tarleton's group used light sheet fluorescence microscopy to view intact whole organs from infected mice. "With light sheet fluorescence microscopy, you have a broad view of potentially any tissue in the mouse that allows for dependable assessment of parasite load and persistence," said Tarleton. "It gives you an incredible view of the infection." Using this technology, they learned something new about the dormant parasites: Some were still susceptible to drug treatment. This provides hope that new drug therapies could be developed to target these parasites. "Discovery of new drugs should continue," Tarleton said. "We still need better drugs." Co-led by assistant research scientist Juan Bustamante and research professional Fernando Sanchez in Tarleton's research group, the study's findings appear in Science Translational Medicine. Explore further More information: Juan M. Bustamante et al. A modified drug regimen clears active and dormant trypanosomes in mouse models of Chagas disease, Science Translational Medicine (2020). DOI: 10.1126/scitranslmed.abb7656 Provided by University of Georgia Citation: Stronger treatments could cure Chagas disease (2020, October 29) retrieved 29 October 2020 from https://medicalxpress.com/news/2020-10-stronger-treatments-chagas-disease.html This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only. |
| Posted: 28 Oct 2020 12:54 PM PDT LILONGWE, Malawi, Oct. 28, 2020 /PRNewswire/ -- Today the World Health Organization (WHO) announced in this week's WHO Weekly Epidemiological Record that Malawi has been validated to have eliminated lymphatic filariasis as a public health problem. MSD (trade name of Merck & Co., Inc., Kenilworth, N.J., USA), GSK, and the Mectizan Donation Program congratulate President Lazarus Chakwera, the government, and the people of Malawi for this remarkable achievement that has alleviated suffering for millions and highlights the perseverance of many dedicated partners. Malawi is only the second country in sub-Saharan Africa to mark this achievement. Lymphatic filariasis (LF), commonly known as elephantiasis, is a debilitating disease caused by a parasite transmitted to humans through the bites of mosquitoes. According to WHO, elephantiasis is found in 73 countries around the world with an estimated 120 million people infected. Long-term, chronic infection causes damage to the lymphatic system, and severe and irreversible swelling to the limbs, breasts, and/or genitals. These symptoms cause extreme discomfort, disability, and social stigmatization. A WHO resolution to achieve the goal of global LF elimination was passed by the World Health Assembly in 1997. In countries where LF and another parasitic disease called river blindness are co-endemic, WHO recommends co-administering two medicines, albendazole and ivermectin to achieve LF elimination. In 1998, GSK announced the donation of albendazole for the elimination of LF and MSD expanded its donation of Mectizan® (ivermectin) through the Mectizan Donation Program to include the elimination of LF in countries where the disease co-exists with river blindness. In 2017, in support of new WHO guidelines, MSD's donation of Mectizan was once again expanded to provide up to 100 million additional treatments per year through 2025 to support the elimination of LF globally in countries where onchocerciasis is not endemic. Since 1999 GSK has donated 9 billion doses of albendazole through the WHO to support efforts to end LF in 65 endemic countries. MSD has donated over 3.76 billion doses to control and eliminate river blindness and LF. "We celebrate this important achievement with the government and the people of Malawi," said Ms. Carmen Villar, Vice President for Social Business Innovation at MSD. "Lymphatic filariasis is a debilitating, but preventable disease. We are proud to be part of a global partnership focused on its elimination and improving the lives of tens of millions of people." Fiona Smith-Laittan, VP of Global Health at GSK, said: "We congratulate the government of Malawi for this significant achievement in ending suffering caused by LF. GSK is committed to improving global health, and we have partnered with WHO, MSD, governments, and partners for over two decades in the Global Program to Eliminate LF. We continue to be inspired by the dedication of the Ministries of Health, frontline health workers, and communities fighting this debilitating disease." The Mectizan Donation Program's director, Dr. Yao Sodahlon, an expert in tropical diseases who played a critical role in the elimination of LF from Togo stated "I am thrilled to see another country in Africa be validated by WHO for eliminating LF. I congratulate Malawi for this remarkable achievement and commend the Ministry of Health for its steadfast commitment since the LF elimination program began in 2008. The effort to achieve consistently high coverage with Mectizan and albendazole paid off as Malawi becomes the second country in sub-Saharan Africa to eliminate LF." About GSK Our work in global health is science-led, sustainable for our business and focused for impact. Each year over 5 million children die under the age of five, most from preventable diseases and in developing countries. We want to help change that by focusing our science on three of the world's biggest health challenges – HIV, TB and malaria. About the Mectizan Donation Program About MSD SOURCE The Task Force for Global Health; Mectizan Donation Program  Related Links |
| 3P Biopharmaceuticals and SpyBiotech Sign Vaccine Manufacturing Agreement - BioPharm International Posted: 28 Oct 2020 12:00 AM PDT  3P Biopharmaceuticals, and Oxford University spin-out, SpyBiotech, have signed a vaccine contract manufacturing agreement. Contract development and manufacturing organization (CDMO), 3P Biopharmaceuticals, and Oxford University spin-out, SpyBiotech, have signed a vaccine contract manufacturing agreement, it was announced in an Oct. 26, 2020 press release. SpyBiotech's SpyCatcher/SpyTag platform technology enables antigens to be displayed onto virus-like particles with a covalent, irreversible bond in a stable and effective way with specific orientation/epitope presentation and high density. The company states that the technology has broad applicability in vaccine development and has established proof of concept data in viral, bacterial, parasitic diseases, chronic diseases, and cancer. In June 2019, SpyBiotech selected 3P Biopharmaceuticals for the manufacture of a current good manufacturing practice (CGMP) batch of its virus-like particle carrier, with the aim for production to be completed in 2021. Through this latest agreement, 3P Biopharmaceuticals will demonstrate its capacity to perform processes and manufacturing of molecules developed for diverse therapeutic applications. "It is amazing being part of a project which presents an opportunity to develop treatments for a broad range of diseases," said Dámaso Molero, CEO of 3P Biopharmaceuticals, in the press release. "We are helping to support manufacturing which we believe will open the door for pioneering vaccines which could offer a hope for improving the lives of many people. That is extremely exciting." Genevieve Labbe, senior scientist and GMP project manager for SpyBiotech added in the press release, "It's critical to have a trusted partner in place for cell line and CGMP production. SpyBiotech is looking forward to advancing this product and we are delighted to be working with 3P Biopharmaceuticals." Source: 3P Biopharmaceuticals |
| How deadly parasites 'glide' into human cells - Science Daily Posted: 13 Oct 2020 12:00 AM PDT In biological terms, gliding refers to the type of movement during which a cell moves along a surface without changing its shape. This form of movement is unique to parasites from the phylum Apicomplexa, such as Plasmodium and Toxoplasma. Both parasites, which are transmitted by mosquitoes and cats, have an enormous impact on global heath. Plasmodium causes 228 million malaria infections and around 400,000 deaths per year. Toxoplasma, which infects even one third of the human population, can cause severe symptoms in some people, and is particularly dangerous during pregnancy. Gliding enables the Apicomplexa parasites to enter and move between host cells. For example, upon entering the human body through a mosquito bite, Plasmodium glides through human skin before crossing into human blood vessels. This type of motion relies on actin and myosin, which are the same proteins that enable muscle movement in humans and other vertebrates. Myosin has a form of molecular 'legs' that 'march' along actin filaments and thereby create movement. In Apicomplexa, myosin interacts with several other proteins, which together form a complex called the glideosome. The exact mechanism by which the glideosome works is not well understood, among other reasons because the molecular structure of most glideosome proteins are unknown. Yet understanding this mechanism could aid the development of drugs that prevent the assembly of the glideosome and thereby stop the progression of diseases such as malaria and toxoplasmosis. Molecular stilts facilitate gliding Scientists at EMBL Hamburg analysed the molecular structure of essential light chains (ELCs), which are glideosome proteins that bind directly to myosin. It is known that they are necessary for gliding, but their exact structure and role were unknown until now. The researchers now obtained molecular structures of ELC bound to myosin A in Toxoplasma gondii and Plasmodium falciparum using X-ray crystallography and nuclear magnetic resonance (NMR). Their study, published in Communications Biology, shows that ELCs work like 'molecular stilts' -- upon binding myosin A, the ELCs become rigid, and start to act as its lever arm. This stiffening lets myosin makes longer steps, which likely accelerates the parasite's gliding movements. The researchers also investigated the role of calcium, a presumed gliding regulator, in the interaction between ELCs and myosin A. Surprisingly, they discovered that calcium does not influence the structure of ELCs. It does, however, increase the stability of the ELC-myosin A complex. This unexpected result shows that the glideosome architecture still hides many unknowns. "This work has provided the first glimpse of how these organisms move around," says Matthew Bowler, an EMBL Grenoble researcher not involved in this study, who investigates Toxoplasma's strategies to control the immune system after invading cells. "It is fascinating to see new molecular details emerge on how these parasites work outside of the host cell. The beautiful structures show how the motor that drives this motion is put together, and could provide a basis to develop new medicines to treat these diseases," continues Bowler. Maria Bernabeu, who leads research on vascular dysfunction in cerebral malaria at the EMBL site in Barcelona, adds: "Plasmodium passage through the skin is the first stage of human infection. The advantage of targeting Plasmodium at that stage is that only about a hundred parasites are present. Understanding the parasite's gliding motility might help to develop drugs or vaccines that target Plasmodium before it multiplies." Interdisciplinary collaboration The work is a result of interdisciplinary collaboration between structural biologists (Löw group) and parasitologists (Gilberger group) from the European Molecular Biology Laboratory in Hamburg and Centre for Structural Systems Biology (CSSB), as well as scientists from the Bernhard Nocht Institute for Tropical Medicine, University of Hamburg and Martin-Luther-University Halle-Wittenberg. It demonstrates the potential of interdisciplinary collaborations in contributing to our understanding of biological processes and possible future strategies to combat parasitic diseases. "Entering malaria research has been an exciting endeavour -- regular exchange with experts and the interdisciplinary environment helped us to explore the field of parasitology," says Christian Löw. Story Source: Materials provided by European Molecular Biology Laboratory. Original written by Dorota Badowska. Note: Content may be edited for style and length. |
| You are subscribed to email updates from "Parasitic Diseases" - Google News. To stop receiving these emails, you may unsubscribe now. | Email delivery powered by Google |
| Google, 1600 Amphitheatre Parkway, Mountain View, CA 94043, United States | |
Comments
Post a Comment