Trouble in parasite Malaria is fighting back against efforts to eliminate it - The Economist
Trouble in parasite Malaria is fighting back against efforts to eliminate it - The Economist |
- Trouble in parasite Malaria is fighting back against efforts to eliminate it - The Economist
- Woman's Brain Tumor Turns Out to Be Parasite: 'The Good News Is I Don't Have Cancer' - Inside Edition
- Is the brain parasite _Toxoplasma_ manipulating your behavior, or is your immune system to blame? - The Conversation US
- Study may explain mystery of long-term parasitic infections - News-Medical.net
| Trouble in parasite Malaria is fighting back against efforts to eliminate it - The Economist Posted: 29 May 2019 05:00 PM PDT THESE SHOULD be hopeful days for those battling malaria. Deaths from the disease have fallen to around 435,000 a year, from perhaps five times that number a century ago. On May 22nd the World Health Organisation (WHO) declared Algeria and Argentina malaria-free, bringing to 38 the number of countries now officially rid of the disease. Algeria will be regarded as a particular success because it is in Africa. The continent suffered 90% of an estimated 219m cases worldwide in 2017. But two big clouds darken the outlook. One is the stubborn persistence of malaria south of the Sahara. The other is the emergence of new strains of the disease resistant to the available treatments. Fewer Africans are dying from malaria but the estimated number of cases has barely changed since 2011. Ten African countries and India account for 70% of global cases. Numbers in India are falling, but not in the worst-afflicted African countries. Some places, such as Zambia (see article), are trying hard to tackle the disease. But malaria is proving resilient. One reason may be the declining share of families that use anti-mosquito sprays in their homes. Another may be resistance to the insecticides used in bed nets or sprays. And, though about three-quarters of the $3.1bn the world spends to fight the disease each year goes to Africa, funding per person has fallen in recent years in the most malaria-prone countries. Perhaps most important, these countries also have shoddy public-health systems, especially in war zones such as northern Nigeria. Such places are typically not equipped to cope with new treatment-resistant strains of the disease. More than 50 years ago, variants resistant to chloroquine, a past treatment, travelled around the world. And South-East Asia, where those variants appeared, is again suffering local outbreaks incurable by some of the main defences used against the disease, artemisinin-based combination therapies (ACTs). Work at Phuoc Long Hospital in Binh Phuoc province in southern Vietnam, which borders Cambodia, is thus of global interest. The facility's 250 beds serve around 200,000 people. Funds are tight. As officials hold a morning meeting under a golden bust of Ho Chi Minh, Vietnam's first communist leader, a toothless former soldier, still in uniform, pushes his bicycle through a courtyard with peeling paint. Doctors proudly show off new equipment for researching malaria. One reckons the hospital sees only 100-odd cases a year. But the rate of failure for one conventional ACT treatment is already frightening—above 60%, says Professor Hien Tran Tinh of the Oxford University Clinical Research Unit. Two types of malaria parasite most trouble the Greater Mekong Region. Plasmodium falciparum kills the most people globally. Plasmodium vivax is to blame for many of the cases of malaria outside sub-Saharan Africa. Less deadly than P. falciparum, it can linger in the liver after recovery and trigger a relapse. Its debilitating cycles leave victims susceptible to other diseases. Like any living organism subject to sufficient pressure, malaria parasites mutate to survive. In parts of the Mekong, the parasites Anopheles mosquitoes inject into the human bloodstream are resisting conventional treatment. By 2030, the WHO hopes to see malaria eliminated in the region before its resistant parasites spread. ACTs work in two main ways. The artemisinin lowers parasite levels in the body within about three days. A partner drug then works to clear them entirely over time. Resistance can develop to both artemisinin and the partner—and both are failing in some areas. Of the six ACTs most used in the Mekong, three are failing in parts of Cambodia, Thailand, Laos and Vietnam, and two in Myanmar. No one is sure why the Mekong seems to spawn resistance—its tropical climate, forests and rubber plantations are all thought to play a role. It also matters how locals behave. In Africa children and pregnant women are especially prone to malaria. In the Mekong it often affects young workers, sometimes engaged in dodgy practices such as illegal logging. Many fail to seek help quickly. Others turn to traditional healers before coming to clinics. Even when given treatment—which is free in countries such as Vietnam—victims often stop taking long courses of medication too soon. Weak governance is another obstacle. Failures in one country can cause trouble for its neighbours. "Vietnam should have eliminated malaria years ago but it can't because of Cambodia," explains one regional malaria expert. Meanwhile, more careful spending among big donors, such as the Global Fund to Fight AIDS, Tuberculosis and Malaria, a public-private body, means less money for local officials. It is therefore harder to get them to go to the remote areas where people need help. A prevention programme needs both to reduce the number of people bitten by infected mosquitoes and to shorten the time before infected people seek treatment. This requires adequate funding for rural health-care services and outreach programmes. Low-cost, rapid diagnostic tests remain one of the most important tools. Dr Hien slides one across the table in Phuoc Long, saying it is fairly easy and cheap to treat malaria if it is detected in the first three days. After that, "the outcomes are much more uncertain." Real-time mapping platforms then allow authorities to track the disease and prepare accordingly. Thailand has created a notably successful one. Joined-up government makes a big difference. Benjamin Rolfe, who runs an alliance of Asia-Pacific leaders to combat malaria, says 12 Chinese ministries have in recent years held regular meetings on tackling malaria. Not a single indigenous case was reported in China in 2017. If national governments are sluggish, subnational leadership can help. Officials from Binh Phuoc province and Kratie province, its Cambodian neighbour, agreed last year to tackle malaria in a more co-ordinated way. Donors, drug firms and governments of rich countries are all working on multi-drug-resistant malaria. Pedro Alonso, the director of the WHO's Global Malaria Programme, says the pipeline of treatments in research and development is "richer than ever". The Medicines for Malaria Venture, which brings together donors and drug companies to develop new treatments, has had 19 new drugs approved for development over the past two decades and has trained 18,000 health workers. A new pill to treat P. vivax infections, Tafenoquine, may soon be available. It is used in a single dose, rather than as a 14-day course. Hopes are high that patients will take it appropriately. Phuoc Long hospital has a partnership with Novartis, a Swiss drug firm. Trials are being conducted into a new drug to fight malaria and two new ACTs. Dr Rolfe estimates that to register a new drug and conduct trials takes seven years. With drug-resistant malaria already emerging, that is an age. In 2016, $588m went into research and development globally—85% of the annual R&D spending the WHO estimates is needed globally by 2020 to cut both malaria cases and mortality rates by 40% by 2030. The total cost of meeting the goals is put at $6.6bn a year.  Steady funding is essential to eliminating malaria. More than a third of the money spent on the cause around the world passes through the Global Fund. Meetings in October will determine how it spends its cash between 2020 and 2022. The hope is that the Mekong is not forgotten; its cases involving resistance remain dangerous. America provides more than a third of the funds for the global fight, so public-health executives are alarmed that the Trump administration plans to cut its anti-malaria spending by over $100m in 2020. Without political commitment and the cash to match, the world risks a relapse in the fight against malaria. Such backsliding occurred in the 1960s, squandering the progress in the preceding decade against the disease in many countries, including India and Pakistan. The hope is that this time success breeds greater commitment rather than greater complacency. Promisingly, a pilot vaccine programme was launched in April. Over the next three years the vaccine, known as RTS,S will be given out in parts of Malawi, Ghana and Kenya. It is used only on young children and works in perhaps just 40% of cases. Still it could save a lot of lives. Scientists have been struggling for decades to produce a really effective vaccine. The battle to vanquish malaria remains extremely long and arduous. |
| Posted: 30 May 2019 09:14 AM PDT  A terrifying diagnosis turned into a scene from a horror movie when a woman's brain tumor turned out to actually be a parasite. Rachel Palma of Middletown, New York, has recovered and been given clear brain scans since the chilling find, but it was just a year and a half ago when that wasn't the case. The 42-year-old said she began noticing something wrong in January 2018. In addition to having hallucinations, nightmares and insomnia, she said she would forget words, drop things all of a sudden and try to call relatives who were dead or nonexistent. "My episodes were getting more and more bizarre. There were days that I didn't know where I was," Palma told the "Today" show. After several visits to urgent care and even more brain scans and testing, doctors noticed a lesion on the left side of her brain, the area that controls language and behavior in right-handed people. Neurosurgeons at the Icahn School of Medicine at Mount Sinai believed at the time that it was a malignant brain tumor, and told Palma she would need surgery, chemotherapy and radiation. "My husband and I were both in shock and we just wanted it taken care of," Palma said. She scheduled surgery with Dr. Jonathan Rasouli in September, but instead of having a tumor removed, doctors found "this very thin, very well encapsulated thing. It looked like a quail egg," from which a baby tapeworm came out soon after, Rasouli said. Doctors assured it was good news. "We were, like, cheering and clapping. We were so happy," Rasouli told WABC. "When we got in there and saw that it was a tapeworm, we were like, 'Yes!' We were so happy!" They explained parasites are normally removed using antibiotics, but realizing the lesion was not a malignant tumor made it that much easier to treat. "The good news is I don't have cancer," Palma joked. As for the parasite in her brain, she said hasn't traveled to any countries where parasites may be common and hasn't eaten raw pork. Even though doctors can't quite pinpoint where she may have picked it up, Palma said she's happier not knowing. RELATED STORIES Woman Has 6 Brain Surgeries at Same Hospital Where She Was Training to Be a Doctor 'Pathogen Soup' Allowed Amoeba to Eat Man's Brain, Claims Lawsuit Against Water Park Firefighter Who Died of Brain Tumor Gets Emotional Escort Before Organ Donation |
| Posted: 14 May 2019 12:00 AM PDT We're not the same when we get sick. Whether it is sneezing when we get a cold, or ferociously biting people when we get rabies, germs change our behavior. That's because germs need to transmit from one host to another. Consequently, host behavior is usually altered in ways that help the pathogen spread. Rabies, for example, causes infected animals to aggressively bite others because the virus transmits through saliva. But some microbes are more subtle. Toxoplasma gondii parasites, which sexually reproduce only in cats but can infect any animal, hijack the brain and affect the host's behavior. In a turn of events that would make Charles Darwin smile, rats and mice infected with Toxoplasma behave in ways that make them easy prey for cats – exactly where Toxoplasma wants to go. The ability of Toxoplasma to disrupt such basic instincts in rodents is alarming when you consider that one-third of humans also carry this parasite's cysts in their brain. Latent toxoplasmosis in humans has been associated with serious neurological disorders, including schizophrenia, intermittent explosive (rage) disorder and suicide, but has never been shown to be a direct cause. Could the parasite be manipulating people as well? Is there a way we can get rid of this parasite and, if so, would behavior return to normal? I am a microbiologist who has been studying Toxoplasma for over 20 years. Not only have I found the parasite's effects on its host to be endlessly fascinating, I have been trying to identify its vulnerabilities so physicians can better treat this currently incurable lifelong infection. In a collaboration with biochemist Ronald Wek and neuroscientist Stephen L. Boehm II, we have made the surprising discovery that the parasites may not be directly manipulating its rodent host. Rather, the host's immune response to the chronic infection may be to blame. Your brain on Toxoplasma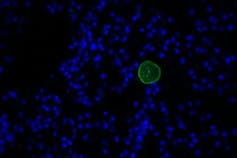 Toxoplasma is a single-celled parasite that really gets around - it has managed to infiltrate the brains of billions of creatures around the world, from birds to beluga whales. Of all the species Toxoplasma can infect, though, only cats support its sexual stage. After sex in the cat gut, Toxoplasma is packaged into sturdy pods called oocysts that are released into the environment via feces, and can then be ingested or inhaled by other animals. Infection with Toxoplasma does not usually produce symptoms in humans unless their immune systems are compromised, but the parasites remain in the body for life as latent tissue cysts. These tissue cysts are commonly found in the brain, heart and skeletal muscle. The formation of tissue cysts occurs in all infected animals, including many that end up on our dinner plate. Consumption of these tissue cysts in raw or undercooked meat also transmits the infection. Another way these tissue cysts serve as a vehicle for parasite transmission is through the alteration of host behavior. Rats and mice with latent toxoplasmosis become hyperactive and lose their instinctual fear of cats, essentially making them a free lunch for felines. Jennifer Martynowicz, an M.D.-Ph.D. student in my lab, was intrigued by the ability of latent toxoplasmosis to alter the behavior of mice. It has long been a mystery as to how exactly this little microbe, which seems inert when encased in its tissue cyst wall, manages to pull off such a feat. It is known that Toxoplasma releases an arsenal of parasite proteins into host cells that can alter gene activity, but how this translates into altering behavior remains unknown. Previous work in our lab found that guanabenz, an FDA-approved drug used to treat hypertension, significantly reduces the number of brain cysts in an infected strain of mice we call BALB/c. Using this drug, Martynowicz was able to address a fundamental question: If we reduce the number of parasite cysts in the brain, can we restore normal behavior?  Toxoplasma changes behavior – drug reverses itMartynowicz administered guanabenz for three weeks to the mice that were hyperactive due to latent toxoplasmosis. When Martynowicz examined the brains of the treated mice and mice that received no guanabenz, she discovered that cyst counts were lowered about 75% in treated mice, reinforcing the results from prior studies. In the first demonstration of its kind, Martynowicz then examined whether the reduction in cysts affected activity levels in the mice. To our delight, the hyperactivity usually seen in mice with latent toxoplasmosis had disappeared. The animals treated with guanabenz behaved like normal, uninfected mice. So it looked like our lab's hypothesis was correct: Brain cysts correlated with behavior changes. To be certain that the hyperactivity was caused by the cysts, Martynowicz decided to examine the effect of guanabenz in a different mouse strain called C57BL/6, which are more susceptible to Toxoplasma. In this mouse strain, guanabenz did not lower cyst counts. But it reversed the hyperactive behavior. These unexpected findings showed that the hyperactivity in infected mice does not correlate with the number of parasite brain cysts after all. To address this puzzling discrepancy, Martynowicz examined the level of inflammation in the brains of these mice. Other investigators have observed that latent parasite cysts in the brain recruit immune cells, producing a low level of sustained inflammation. Is brain inflammation changing behavior?Guanabenz is known to have anti-inflammatory effects. Decreasing brain inflammation is exactly what it appears to be doing in the brains of both infected mouse strains. These results suggest that the hyperactivity in infected mice is more likely driven by their immune response rather than a parasite-driven manipulation. If so, the key to controlling some behavioral changes in infected animals may be modulating their immune response. 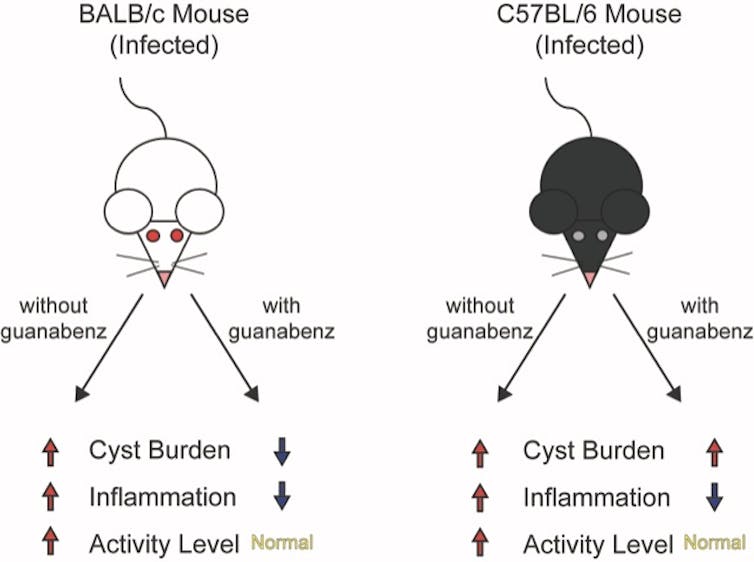 We do not yet know how neuroinflammation may lead to hyperactivity. But it is interesting to note that some emerging studies have also found a link between inflammation and attention-deficit hyperactivity disorder (ADHD), which affects more than 6 million children in the U.S. If our findings in mice, published in the journal mBio, extend to people, it could have important ramifications for how we currently treat brain infections. Our results suggest that brain infections may cause neurological consequences only in a subset of people, based on their immune response. Further studies are needed to determine if anti-inflammatory drugs like guanabenz may be effective at managing these conditions. |
| Study may explain mystery of long-term parasitic infections - News-Medical.net Posted: 22 May 2019 12:00 AM PDT  As hosting gigs go, it's a tough crowd. More than 1 billion people are host to parasitic worms that take up residence in their intestines. For most, it's a short stay, with the immune system evicting the worms in days or weeks and leaving no trace that the parasites were ever there. In a small percentage of people and other animals, though, the worms gain a permanent mouth-hold and can stick around the intestines for years. That persistence often leads to malnutrition, which in turn tightens the grip of infection, initiating a spiral that becomes difficult to escape. The question of why that small percentage struggles to expel parasites has stymied researchers for a while. But a new mathematical model developed at the University of Nebraska-Lincoln and Royal Netherlands Institute for Sea Research offers an answer while reinforcing an adage: safety in numbers. Clay Cressler, Anieke van Leeuwen and their colleagues have concluded that when large numbers of worms stake out intestinal territory in a host, they generally manage to hang in there for the long term. By contrast, the model suggests that lone worms -- or small groups of them -- get overwhelmed by the immune system and quickly flushed from the GI tract.
The team's model can simulate host-parasite interactions by accounting for multiple variables: number of parasites, availability of food, body mass of the host, how much of that mass is accessible to parasites vs. the immune system. Crucially, it also factors in that parasitic worms can manipulate a host's ability to digest food and mount an immune response. As part of a recent study, the team simulated whipworm infections for 1,000 mice while varying the number of worms that infected their colons. From those results, the researchers deduced that a large collective of whipworms can kick-start a self-perpetuating cycle that nearly guarantees the parasites' survival. Though the specifics aren't totally clear, prior research has led the team to suspect that a critical mass of worms may unleash a barrage of molecules that confuse or suppress the immune system and allow the parasites to begin high-jacking a host's nutrients. "Anything that a parasite does to increase its own access to resources indirectly deprives the host of them," Cressler said. "The more parasites there are, the better they are at manipulating resources, and the better they are at manipulating, the better the parasites grow. And then they're even better at manipulating. So you get this positive-feedback loop that allows them to establish a chronic infection." When a moderate number of worms infect a host, their length of stay becomes less certain and seems to depend more on other factors: How large are the host's fat reserves? Has its immune system already been primed to respond? How much is it eating? On the latter question, the team found evidence that more food increases the odds of a long-term stay. "Historically, the perspective is that food really only matters for the host," Cressler said. "Only recently, within the last 10 years or so, has there been more of a focus on what diet is doing to the parasite. Is there a chance that what you're actually doing is just feeding the parasite more? In this case, that's what happens. "If (biological) systems are frequently in this place where you can go either way, then small changes in other things that we're not (historically) paying attention to can be the things that determine whether you go one way or the other." Yet when all other factors are equal, the model indicates that the quantity of parasitic worms alone may determine whether they persist in a host. That's a potentially important advance, Cressler said, given that prior approaches have mostly treated parasite duration as a fixed, independent variable rather than one dependent on other factors. And when the team compiled the fates of each mouse in its simulation, the resulting distribution resembled the pattern commonly seen in nature: most hosts burdened with just a few parasites, a few hosts burdened with many. "Most of the theory that we use in understanding immune-parasite interactions is insufficient to address the question of where this kind of variation comes from," Cressler said. "If you have this ability to (allow for) either long duration or short duration, what you end up seeing are many hosts who keep getting the short-term infections and have very low burdens, and some hosts who will keep getting these long-term infections and end up with a very high burden. "So this hypothesis could potentially help to explain two big patterns in parasitology: this widespread variation in how long infections last and this widespread variation in how sick each host is." Though the team's model first needs to withstand the scrutiny of experimentation and further research, Cressler said, it might eventually help inform the treatment of parasitic infections or calibrate predictions of chronic cases. Doing so before potentially chronic infections result in malnutrition -- and diminish the usefulness of drugs that otherwise clear parasites -- could prove especially valuable in the developing world. "If we could (eventually) tell you, 'This is the stuff you need to measure to know whether somebody is going to be chronically infected,' then that might tell you something about how you need to treat or who you need to treat," Cressler said. "Where's the highest-risk area, where the environment is such that people are going to be primed toward this chronic rather than acute infection? "This variation in duration is a real pattern, and it has real consequences for people. Anything we can do that helps to understand how these infections work could potentially have a therapeutic value later. We don't know what that is now. But that's the goal." Source: University of Nebraska-Lincoln Journal reference: Cressler, C. et al. (2019) Parasite resource manipulation drives bimodal variation in infection duration. Proceedings of the Royal Society B. doi.org/10.1098/rspb.2019.0456. |
| You are subscribed to email updates from "How do you treat parasites" - Google News. To stop receiving these emails, you may unsubscribe now. | Email delivery powered by Google |
| Google, 1600 Amphitheatre Parkway, Mountain View, CA 94043, United States | |
Comments
Post a Comment